This post was published by Jon Fleetwood. Please visit his Substack and subscribe to support his work. Follow Jon: Instagram@realjonfleetwood / X@JonMFleetwood / Facebook@realjonfleetwood
The chemical used in the Trump administration’s new ‘Generation Gold Standard’ bird flu vaccine platform—beta-propiolactone (BPL)—is classified as a ‘Group 2B’ carcinogen by U.S. regulators (meaning the substance is possibly cancer-causing in humans) and as a ‘Group 1B’ carcinogen in Europe (meaning it’s presumed to cause cancer in humans).
A carcinogen is any substance or agent capable of causing cancer by damaging a cell’s DNA and leading to uncontrolled cell growth.
The revelation follows this website’s report that the U.S. National Institute of Allergy and Infectious Diseases (NIAID)—under Dr. Jeffery Taubenberger’s direction—has been funding the creation of chimeric, lab-engineered bird flu viruses in American and foreign laboratories, even as Taubenberger himself is named on a federal patent for the very BPL-utilizing bird flu vaccine platform those experiments are designed to justify.
A May 2025 government press release announced the multi-million dollar BPL platform will be used to generate bird flu pandemic vaccines:
This initiative represents a decisive shift toward transparency, effectiveness, and comprehensive preparedness, funding the NIH’s in-house development of universal influenza and coronavirus vaccines, including candidates BPL-1357 and BPL-24910. These vaccines aim to provide broad-spectrum protection against multiple strains of pandemic-prone viruses like H5N1 avian influenza and coronaviruses including SARS-CoV-2, SARS-CoV-1, and MERS-CoV.
The U.S. Department of Health and Human Services (HHS) and the National Institutes for Health (NIH) today announced the development of the next-generation, universal vaccine platform, Generation Gold Standard, using a beta-propiolactone (BPL)-inactivated, whole-virus platform.
U.S., UN, WHO, IARC ‘Group 2B’ Classification: Possible Carcinogen in Humans
Since 1974, the International Agency for Research on Cancer (IARC), an intergovernmental agency of the United Nations’ (UN) World Health Organization (WHO) that researches the causes of cancer, has known that BPL causes cancer in mammals.
According to IARC documents, “?-Propiolactone was tested for carcinogenicity in mice following skin application or subcutaneous or intraperitoneal injection, and in rats following subcutaneous injection, producing local tumours. It is carcinogenic to mice after single-dose exposure. Oral administration to rats gave some indication of carcinogenic activity. The results obtained in Syrian hamsters and guinea-pigs are equivocal (IARC, 1974).”

Pp1079 1142
105KB ? PDF file
A February 2020 peer-reviewed study in ACS Publications, the publishing division of the American Chemical Society (ACS), confirms BPL is likely carcinogenic in humans and causes death in animals.
“Direct exposure to BPL, in addition to probable carcinogenicity, brings severe irritations to several systems, including skin burns and permanent damage to the eye, liver, and kidney. Even at a single administered dose, BPL is shown to be highly tumorigenic, genotoxic, and carcinogenic in experimental animals. At higher doses it even causes death,” the study reads.
The U.S. Centers for Disease Control and Prevention (CDC) confirms BPL is a “potential occupational carcinogen” because the compound causes “tumors of the liver, skin & stomach” in animals.
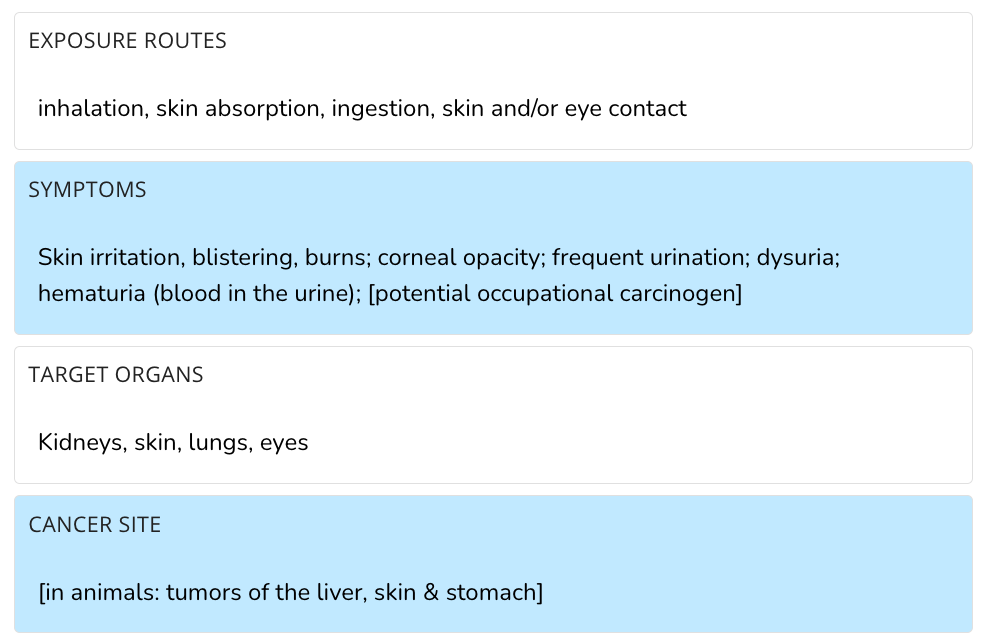
Documents from the U.S. Environmental Protection Agency (EPA), an independent federal agency responsible for protecting human health and the environment, acknowledge that BPL is “classified …as a Group 2B, possible human carcinogen” by the IARC.

The New Jersey Department of Health and Senior Services also confirms the following regarding BPL:
- “should be handled as a carcinogen—with extreme caution”;
- “is on the Special Health Hazard Substance List because it is a carcinogen, mutagen and corrosive”;
- “[t]here may be no safe level of exposure to a carcinogen, so ill contact should be reduced to the lowest possible level”;
- “may be a carcinogen in humans since it has been shown to cause skin and stomach cancers in animals”;
- “[m]any scientists believe there is no safe level of exposure to a carcinogen. Such substances may also have the potential for causing reproductive damage in humans”;
- “Most scientists agree that a chemical that causes cancer in animals should be treated as a suspected human carcinogen unless proven otherwise.”

The U.S. Health and Human Services’ (HHS) National Toxicology Program (NTP) also confirms that BPL “is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.”

The U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA) confirms (here) that BPL is “identified as potential occupational carcinogen.”

European ‘Group 1B’ Classification: Presumed Carcinogen in Humans
The European Chemical Agency (ECHA) designates (here) BPL with the more dangerous Group 1B carcinogen classification, where a substance is seen as not only possibly causing cancer in humans, but is “presumed” to have carcinogenic potential for humans.
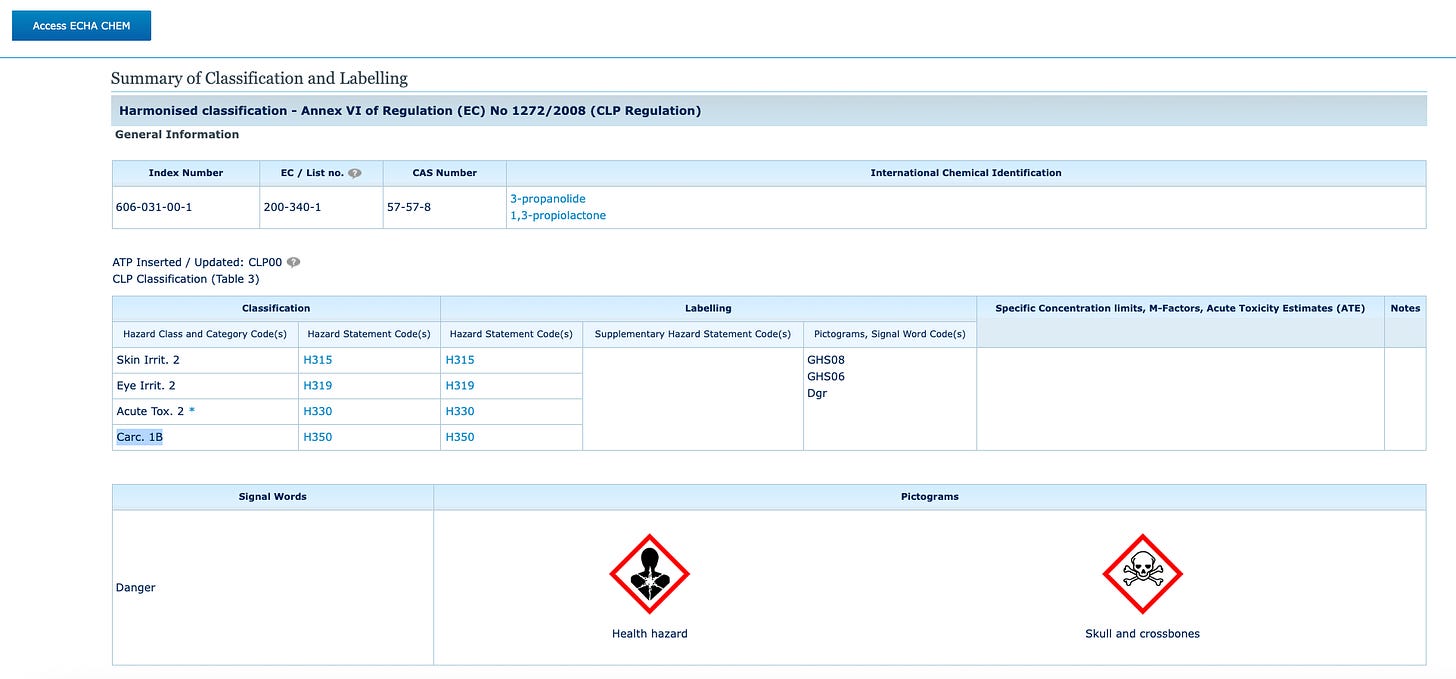

European chemical manufacturer safety data sheets confirm BPL’s 1B carcinogen classification, even requiring a skull and crossbones table.

Even ‘Trace’ Amounts of BPL Are Dangerous—But Vaccines Contain Detectable Amounts
An October 2025 publication in the peer-reviewed journal Analytical Methods, published by the U.K.’s Royal Society of Chemistry, confirms that even residual amounts of BPL in vaccine vials are “unacceptable.”
The authors explicitly warn that it is “necessary to eliminate any trace amounts of BPL remaining after the inactivation process to ensure vaccine safety.”
“One of the most commonly used techniques for producing vaccines is viral deactivation,” the study reads. “?-Propiolactone (BPL) is an agent used to inactivate and sterilize biological products, including vaccines. BPL is favored for its ability to preserve virus capsid proteins and maintain high immunogenicity. However, BPL is an alkylating agent with potential carcinogenic properties, making it unacceptable for residual amounts to be present in the final vaccine product. Therefore, it is necessary to eliminate any trace amounts of BPL remaining after the inactivation process to ensure vaccine safety.”

An August 2024 study published in the peer-reviewed Journal of Chromatography B affirms that due to BPL’s carcinogenicity, “its residual should be hydrolyzed completely in vaccines to avoid cytotoxicity.”
Like the October 2025 Analytical Methods publication, this means BPL must be completely chemically broken down before the vaccine is safe.
However, vaccines utilizing BPL do contain trace amounts of the carcinogenic compound.
For example, an FDA document for the Imovax® Rabies Vaccine produced by Sanofi Pasteur SA confirms BPL “is present” in the final vial before being injected into patients.
“The Imovax® Rabies Vaccine produced by Sanofi Pasteur SA is a sterile, stable, freeze-dried suspension of rabies virus prepared from strain PM-1503-3M obtained from the Wistar Institute, Philadelphia, PA,” the insert reads.
“The virus is harvested from infected human diploid cells, MRC-5 strain, concentrated by ultrafiltration and is inactivated by beta-propiolactone. One dose of reconstituted vaccine contains less than 100 mg human albumin, less than 150 mcg neomycin sulfate and 20 mcg of phenol red indicator. Beta-propiolactone, a residual component of the manufacturing process, is present in less than 50 parts per million.”

Canadian government fact sheets for Imovax confirm immune system disorders linked to the vaccine “might be associated with the presence of betapropiolactone-altered human albumin in the Human Diploid Cell Rabies vaccine (HDCV).”

Bottom Line
The federal government’s new Generation Gold Standard vaccine platform is built on a chemical that regulators across the U.S., Europe, and the U.N. agree can cause cancer.
Beta-propiolactone (BPL) isn’t a fringe concern.
It’s a known carcinogen that produces tumors in multiple animal species and is officially listed as a Group 2B possible human carcinogen by U.S. agencies and a Group 1B presumed human carcinogen in Europe.
Even trace residues of BPL are considered “unacceptable” in finished vaccines by peer-reviewed toxicology standards.
Yet FDA-approved products like Sanofi’s Imovax® Rabies Vaccine acknowledge its detectable presence.
That means the same federal agencies engineering new pandemic pathogens and vaccines are sanctioning the injection of a substance long recognized as capable of inducing cancer, genotoxicity, and organ damage.
In short, the U.S. government’s flagship “next-generation” bird-flu vaccine platform depends on a chemical that, by its own agencies’ definitions, should never be inside the human body.
Your support is crucial in helping us defeat mass censorship. Please consider donating via Locals or check out our unique merch. Follow us on X @ModernityNews.
More news on our radar
















Voting for a non-conservative candidate or by name only causes cancer.
I have made 200 USD reliably in one day.That was my ideal day in my life and my boss was to a great degree content with me..T49 CNN is additionally awed from my work and is outstandingly happy..check also unpretentious parts by open the affiliation and tap on HOME TECH OR
MEDIA……… tab on my name